The medical industry uses Additive Manufacturing (AM) to create products at scale including 3D-printed anatomical models, surgical guides, prosthetics and implants, and even personalized drugs. They benefit from the same features that additive manufacturing brings to other industries such as automotive and aerospace — lighter, easier-to-produce parts at a fraction of the cost.
AM streamlines Product Lifecycle Management (PLM) including reducing prototype costs and getting to market faster. This ushers in huge benefits for patients.
“We work on all sorts of things, but the medical devices are very special to us because they impact someone’s life at the end of the day. And to see that product go from a concept all the way through the design process and get launched in the market and then actually be impacting someone’s life is very rewarding.”
-Casimir Sienkiewicz, Founder of Caztek Engineering
The companies at the forefront of using AM to streamline their product lifecycle management for medical devices include companies like Stryker, Johnson & Johnson, and Cardinal Health. Some of the most important use cases for medical 3D printing include rapid prototyping, point-of-care solutions, serialization for regulatory compliance, and even things like ensuring no disruptions in production will occur like supply chain constraints. “Many companies turned to 3D printing of production parts to keep their supply chains flowing during the COVID-19 crisis, some 71% of manufacturing companies attempted to use 3D printing to address supply chain issues, according to a Dimensional Research Study.”
– 2023 SME AM Survey and Industry Report.
AM for medical helps to shorten lead times, reduce carbon emissions, and bring more control over production in-house.
“AM is also reducing production times and inventory levels across the value chain, leading to cost savings for healthcare providers. All of which continue to warrant investment into AM by leading medical companies.”
-2023 SME AM Survey and Industry Report
What’s the Return on Investment for the Medical Device Industry When It Comes to 3D Printing and Scanning?
R&D on a tighter timeline: Medtronic estimates they’ve saved about nine years on R&D due to their adoption of additive manufacturing.
Save critical time: Summer Decker, PhD Vice Chair for Research and Director of 3D Clinical Applications at the University of South Florida Morsani College of Medicine presented one use case where OR time for one surgery was reduced to 3 hours for a surgery that typically takes 11 hours in this presentation on 3D printing for medical. With things like 3D-printed customized prosthetic limbs and surgical implants, parts can be turned around in as little as 24 hours.
Bring down costs when you bring manufacturing in-house: For 3D printing for dentistry, one estimate suggests that dental professionals who go in-house with 3D point-of-care solutions save on average over a hundred dollars per bridge in lab fees alone.
Reduce costs due to product recalls: Additive manufacturing can also help detect design flaws earlier in the product lifecycle, minimizing the risk of recalls which can cost millions, if not billions, depending on the product.
What is the Product Lifecycle like for Medical Devices with the Addition of Additive Manufacturing?
At a high level, the product development lifecycle includes:
- Concept and prototype: Concepting the device, creating designs, and making a well-researched working prototype backed by customer input and insight from the competitive landscape.
- Validation: Testing and validating the design and verifying the part(s) will work as intended with an emphasis on safety, and risk management.
- Manufacturing: Building the device and designing a workflow for creating the medical device at scale.
- Marketing: Creating regulatory-compliant marketing messages with well-documented research and data to back up claims.
- Getting to market: Launching your medical device, gathering customer feedback and insights through post-market surveillance.
Depending on the type of device you’re making, regulatory approvals from agencies like the FDA or the MDD may happen at various stages in the product development process.
How does HRS help product lifecycle management for medical device manufacturers?
With the right software medical device manufacturers can reduce prototype costs, optimize and validate design, reduce waste, uncover design flaws, enhance collaboration, and, ultimately, get to market faster.
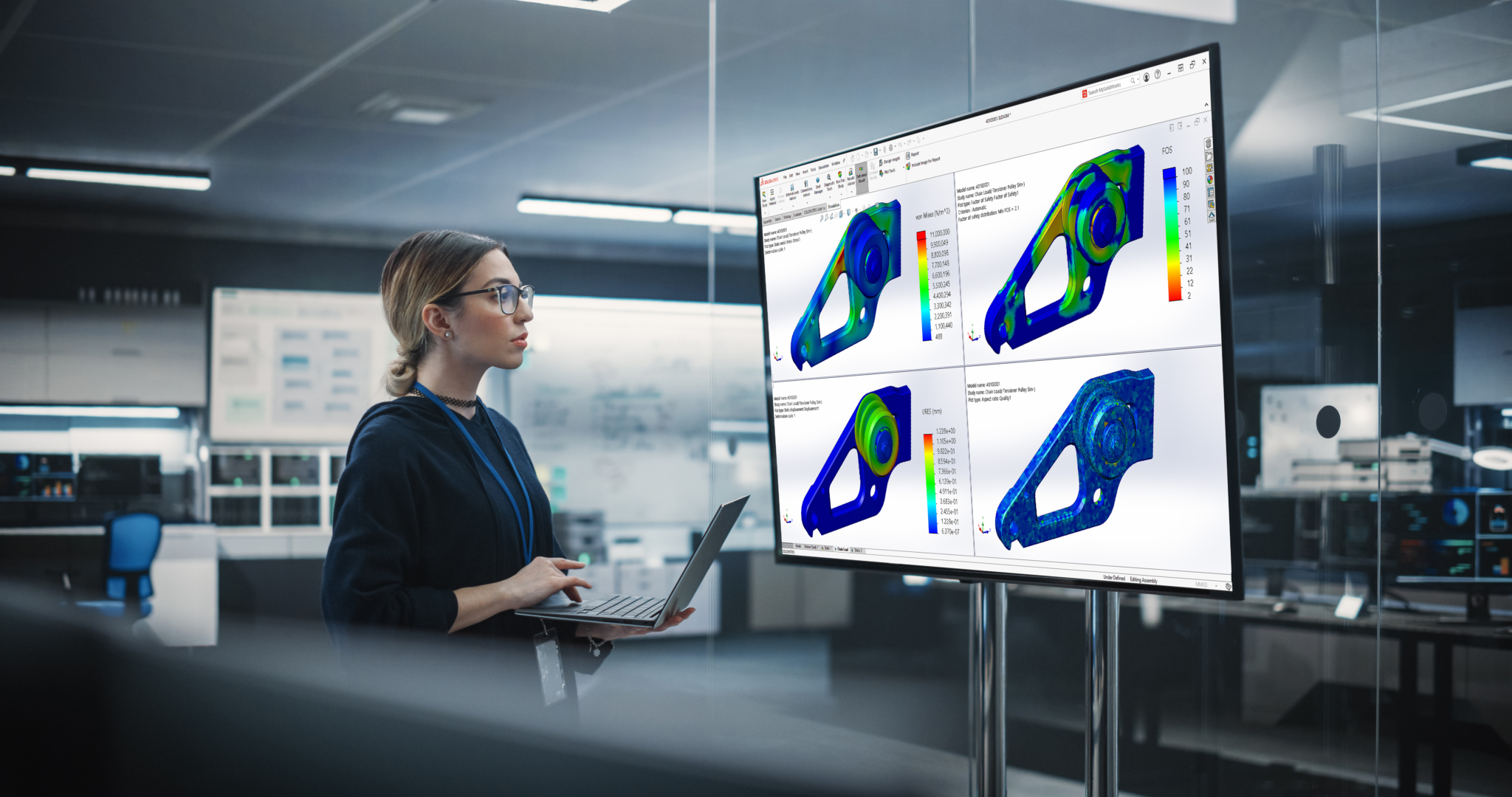
Reduce prototype costs: Engineers can simulate the performance, behavior, and functionality of a medical device part before physical prototypes are even created. Medical device design can be sped up through simulation software.
Drive efficiency and ensure compliance: Streamline and automate your documentation across your entire organization for FDA audits and regulatory requirements with our PDM and PDL data solutions.
Understand which materials work the best: Engineers can test out different materials to verify what the parts or product should be made from, with a focus on minimizing material usage and seeing which materials are safest and most structurally sound.
Test and validation: Engineers can see how a part responds under stress sources such as heat, fluid flow, and vibration to failure points, and uncover opportunities to improve the design. These simulations reduce the time it takes to get a product to market.
Collaborate between stakeholders: Engineers, designers, and stakeholders can communicate and collaborate more effectively through the use of 3D simulation software, reducing rework and miscommunications.
Get to market sooner: Engineers have faster development cycles with AM tooling like Simulia because they can iterate through design variations more quickly.
What Types of 3D Software Do Medical Device Manufacturers Utilize?
Advanced FEA software for medical stint designers
With our cutting-edge Advanced FEA software (Abaqus / 3DX FEA), tailored for Medical Stent Designers, you can create superior stents made of super elastic metal materials. This specialized FEA solution produces accurate and reliable simulations for stent performance in the human body so you can produce safe, effective stents for diverse patient scenarios.
Flow simulation software for understanding fluid behavior in medical devices
With our powerful flow simulation software, you can get unparalleled insights into fluid dynamics, enabling you to streamline and enhance your products and procedures. Our customers using this tool get better and more reliable lab results and improved oxygen delivery by optimizing the design of oxygen masks. Our customers have also increased the performance of inhalers, and improved surgical outcomes for patients by addressing thermal challenges in the operating room.
Electromagnetic simulation software for medical wearables
Our customers leverage SIMULIA CST to develop lifesaving injectables like pacemakers, medical wearables and complex machinery such as MRI machines, while adhering to SAR (Specific Absorbed Radiation) compliance requirements. SIMULIA CST gives your team information about the behavior of optimized injectables so you can increase the performance, reliability, and safety of the products you’re developing. For MRI machines, you can stimulate and analyze electromagnetic interactions to improve performance. SIMULIA CST also enables our customers to streamline their product development process for SAR compliance so regulatory requirements can be met without compromise.
What Types of 3D Hardware Do Medical Device Manufacturers Utilize?
Formlabs Fuse 1
Romans Ferrari, a pediatric rehabilitation center, used scanners and the Formlabs Fuse 1 to create customized compression masks to help heal scars for burn victims. Some key benefits of Formlabs Fuse 1 are that you can create 3D-printed biocompatible orthotics and prosthetics that adhere to regulatory requirements with uncompromising quality.
Artec Scanner
One example of how medical device companies are using the Artec Scanner is to make prostheses for amputees. Custom plaster molds could take up to eight hours to produce, but with the Artec Scanner, scans can be created in a matter of minutes. The Artec Scanner provides advanced scanning technology which empowers surgeons and healthcare professionals to achieve unparalleled levels of accuracy and customization in surgical procedures. Key benefits of using the Artec Scanner include enabling surgeons to plan and execute procedures with confidence, creating customized patient-specific solutions like 3D printed implants and surgical guides, and drastically reducing time for surgical planning and preparation by completing scans and captures quickly and accurately.
Why choose Hawk Ridge Systems as your partner?
Streamlined Compliance & Data Security Solution
At Hawk Ridge Systems, all our tooling is built with compliance in mind, and we have deep expertise and history with a strong medical industry customer base using our products in their daily operations — companies like Align, B Braun, and Johnson & Johnson. Safeguarding sensitive medical data is paramount in the design and manufacturing process, which is why we have some of the most advanced additive manufacturing products on the market such as PDM and Advanced SIM.
Collaboration and Expert Guidance for Optimal Results
We can help you accelerate your product development lifecycle with 3D printing and scanning by breaking down silos between design and manufacturing. We do this through tools like the 3DEXPERIENCE Platform, but also through the services we provide. Hawk Ridge Systems also offers consulting and guidance on your workflows and best practices in the industry.
End-to-End Nationwide Support
Our support team ensures you get the most out of your purchase — from implementation to training and on-going maintenance, your dedicated support team will ensure a seamless experience that will help you streamline your product development lifecycle. Every medical device project is unique, which is why our services are too, meeting your specific requirements, in your time zone.
Questions about Additive Manufacturing for Medical? Contact us at Hawk Ridge Systems.
Sources
2023 SME AM Survey and Industry Report. Accessed August 2023.
Ackuretta | 3D Printing Resin for Dental Crown – CURO Crown. Accessed August 2023.
Casimir Sienkiewicz, Founder of Caztek Engineering. Accessed August 2023.
Device Approvals, Denials and Clearances, U.S. Food & Drug Administration. Accessed August 2023.
Medical 3D Printing Lab Showcase. Accessed August 2023.
Medical Devices Directorate, Canada. Accessed August 2023.
Medtronic LinkedIn Post. Accessed August 2023.