When producing parts for medical applications, the topic of sterilization comes up quite often. Can 3D printed parts be sterilized using traditional methods? What are the effects of sterilization methods on 3D printed parts? We are going to provide insight into these questions and more with a focus on PA12 material off an HP Jet Fusion 3D printer. HP printers like the 5200 are perfect for manufacturing large batches of parts and they have been heavily utilized during the pandemic to fill gaps in supply changes for many devices. PA12 material from HP has been certified for biocompatibility meeting USP Class I-VI and US FDA guidance for Intact Skin Surface Devices, making it an excellent choice for certain medical applications.
To answer the first question, yes PA12 parts off an HP 3D printer can be sterilized successfully. Although the method and type of sterilization will yield different results, especially when considering mechanical properties and moisture absorption. Let’s start by looking at a few sterilization methods that have been used on HP printed parts.
Proven Sterilization Methods for HP PA12 Parts
Autoclave (Steam Sterilization): Process of subjecting parts to steam at elevated temperatures and pressures between 121-148 °C at 200-300kPa until microorganisms are destroyed. This is one of the most popular sterilization methods out there. Autoclave sterilization is the most common method we see our customers utilizing for end-use parts in medical applications. It should also be noted that lower temperature methods are preferred for minimal impact on the performance of the part. Steam sterilization methods have been known to produce splotches/watermarks on processed samples from humidity absorption into the part. This splotching and moisture absorption can be mitigated by vapor smoothing parts before sterilization.
Here are some common test parameters for autoclave sterilization:

Formaldehyde – Low-temperature sterilization method using formaldehyde gas combined with sub-atmospheric steam. (~60°C is common)
Gamma Irradiation – Exposure of samples to ionizing radiation (gamma) from a radio isotopic source (ex: Cobalt 60). Normal radiation levels are 25kGy-50kGy.
Electron Beam (EB) – Irradiation method which uses high-energy electrons to bombard samples. This method has shorter treatment times compared to gamma radiation and uses lower energy radiation. E-beam has lower penetration than gamma and is limited to being used for smaller, less dense parts.
Sterilization Effects on PA12 MJF Printed Parts
We have obtained testing data carried out by HP and independent medical facilities using sterilization data on dogbone samples approximately 170mm x 20mm x 4mm. Below represents a summary of the results and effects on mechanical properties. Please keep in mind that these numbers and test data should only serve as a general reference. For the best data, it is encouraged to carry out your own testing internally.
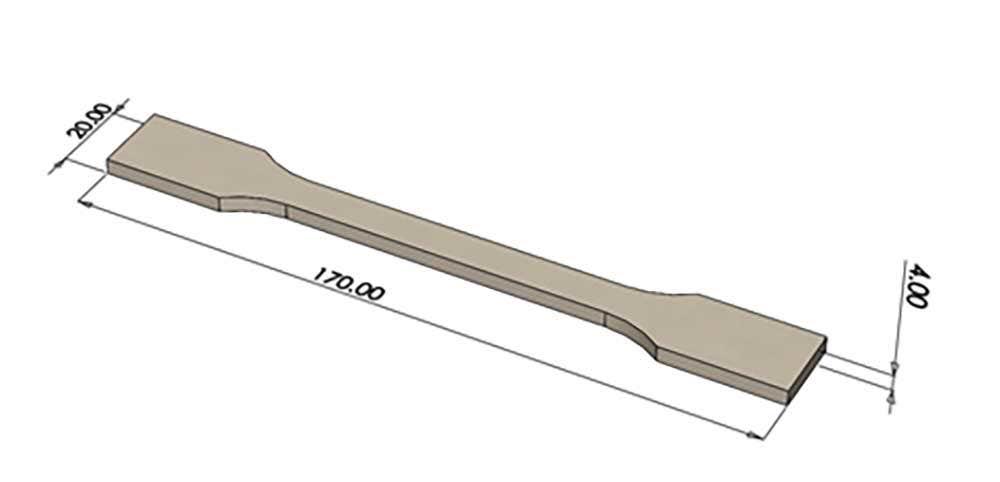
Mechanical test results:
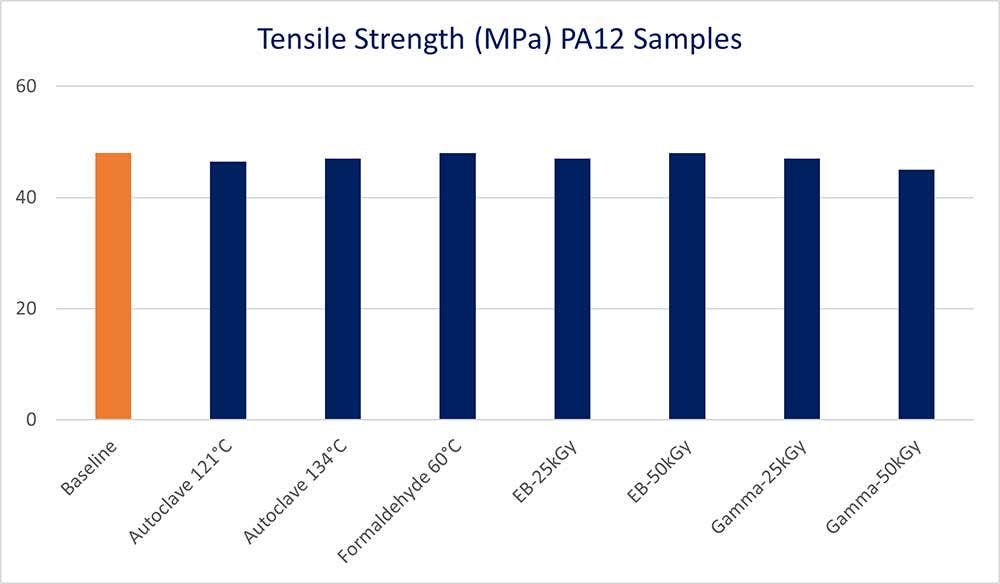
Tensile Strengths remained mostly unchanged after each sterilization process.
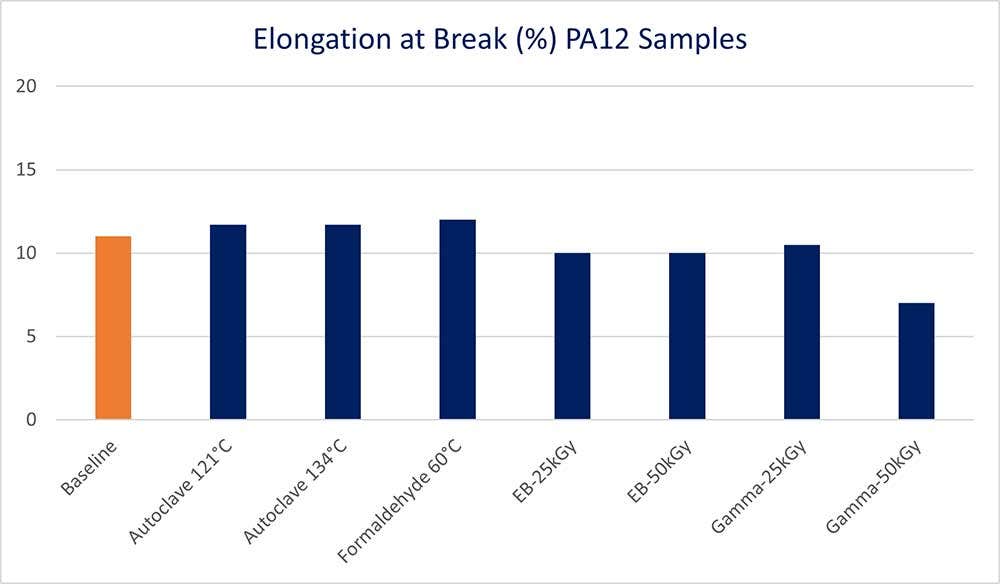
Samples that underwent irradiation sterilization lost elongation at break, lowering the overall ductility of samples.
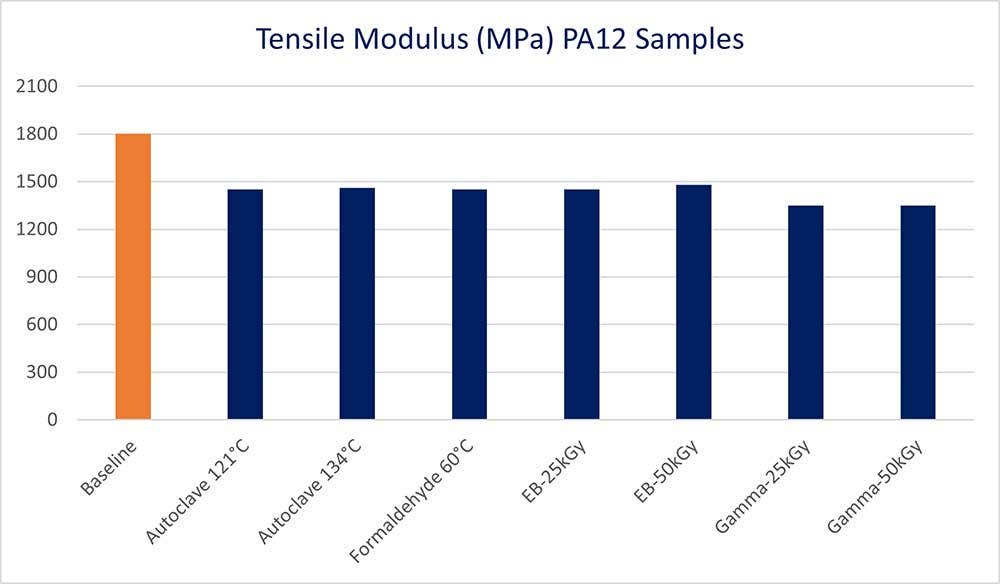
Across all of the sterilization methods tested, the most impacted mechanical property was a lowering of the tensile modulus by roughly 20 percent. This results in a loss of stiffness.
Additional Tests
Additional testing was conducted on different geometries to indicate dimensional variation, warpage, and mass change experienced from sterilization methods.
Dimensional Variation
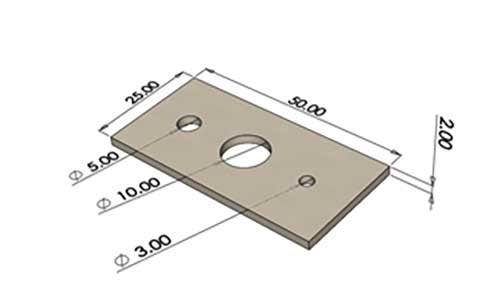
Rectangular samples sized 50mm x 25mm x 2mm with 3 holes were tested for dimensional variation. The diameters of the holes were 5mm, 10mm, and 3mm there was less than 0.015mm change in the diameter after samples went through each sterilization method. This essentially proves that these sterilization methods have a negligible effect on the dimensions of parts in any meaningful way for PA12 samples.
Warpage
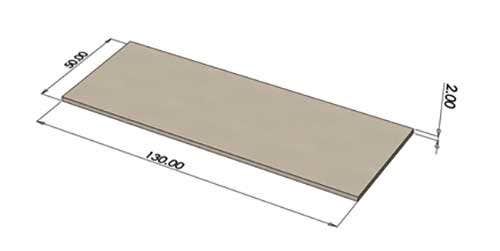
Warpage was tested on a thin flat rectangular geometry measuring 130mm x 50mm x 2mm.
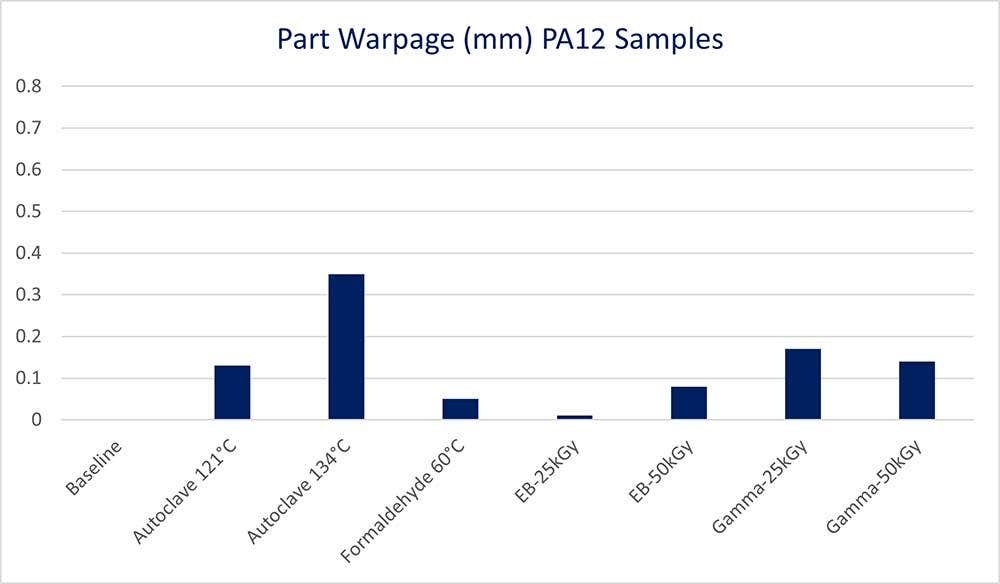
It can be observed from the data that higher temperature methods (excluding irradiation samples) have a greater effect on the overall planar warpage of a sample. Choosing the lower temperature method is advisable, when possible, especially for Autoclave samples.
Weight
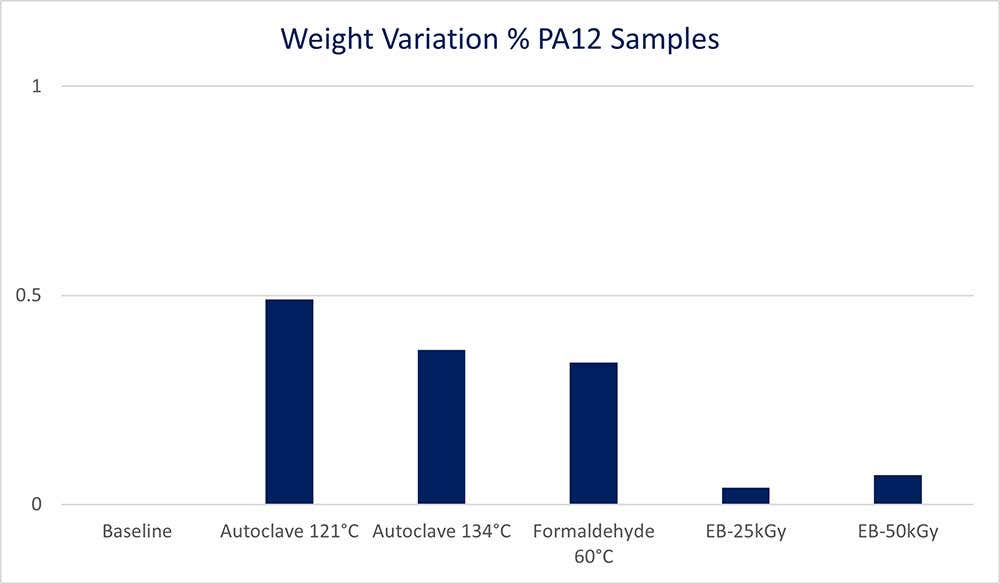
Irradiation methods show to have the least effect on mass variation within samples while non-irradiated samples gained the most mass. The mass change is likely a result of trapped humidity on the non-irradiated samples. Vapor smoothing would be recommended postprocessing technique to improve the performance of samples in this test to mitigate moisture absorption into the part. To learn more about vapor smoothing, check out our blog, “Advantages of PostPro Vapor Smoothing for HP 3D Printed Parts.”

Hopefully, this data and information will serve as a useful guide as you plan out printing for medical applications. It is always encouraged to verify this test data with your own internal testing practices. If you would like to know more about sterilization methods for HP Jet Fusion 3D printed parts, feel free to contact us at Hawk Ridge Systems today!